In 2017, some 10 million people suffered from tuberculosis and 1.6 million died of the disease. One reason why infection with Mycobacterium tuberculosis is so difficult to treat is that the bacteria can hide inside immune cells. University of Groningen scientists, together with a team from the Division of Rheumatology, immunology and Allergy led by professor D. Branch Moody at Harvard Medical School and several other colleagues, have now discovered a key mechanism in the bacteria which prevents the immune cells from killing them: the bacteria produce a unique type of antacid which gives the immune cells indigestion. The results were published in Nature Chemical Biology on 19 August 2019.
KNCV Tuberculosis Foundation congratulates the researchers on their results; it is very important that this type of research is continued and funded. These new insights could lead to the development of new medication for tuberculosis in the longer term.
Invading bacteria are gobbled up by immune cells called macrophages. They encapsulate the intruders inside a phagosome, a vesicle which then fuses with another vesicle full of enzymes, the lysosome. After this fusion, the enzymes break the bacteria down. But not in the case of Mycobacterium tuberculosis: ‘They can survive for years inside a macrophage, where antibiotics can barely reach them’, explains University of Groningen chemist Jeffrey Buter, first author of the paper. He has worked for years on tuberculosis in Groningen under the supervision of Professor Adri Minnaard, as well as at Harvard Medical School, supervised by Professor David Branch Moody. Both supervisors are joint lead authors of the new paper.
Virulence
In a previous study published in 2014, the team identified lipids present in M. tuberculosis but not in M. bovis, a bacterium which is much less virulent. The lipids specific to M. tuberculosis could play a role in virulence. Indeed, an important candidate was found and identified as 1-tuberculosinyladenosine (1-TbAd), an adenosine modified by the attachment of a lipid in the 1-position. ‘Such a modification is extremely rare in nature’, says Buter, ‘yet M. tuberculosis produces and releases a relatively large amount of this compound’.
Two enzymes critical to the production of 1-TbAd were identified, but the mechanism by which this molecule helped the tuberculosis bacterium to survive remained a mystery. ‘Then, we found research performed by another group in 2004, in which it was shown that the fusion of the phagosome and lysosome was blocked due to these enzymes. As the phagosome needs to be acidic for fusion, this led us to the hypothesis that 1-TbAd played a role in preventing acidification of the phagosome’.
Antacid
‘1-TbAd is a weak acid and in equilibrium with its basic counterpart’, continues Buter. ‘In the acidic environment of the phagosome, this base will reduce acidity’. This finding suggested that the molecule works as an antacid and prevents the phagosome from reaching the level of acidity required to fuse with the lysosome.
The group performed a series of experiments to rule out the possibility that the adenosine compound acted through a receptor. The findings confirmed that 1-TbAd does indeed work by reducing the acidity directly. Buter synthesized different variants of the molecule to determine which parts of the molecule were vital for its function. ‘The lipid part is needed to cross the membranes and get into the phagosomes and lysosomes’, says Buter.
Malaria
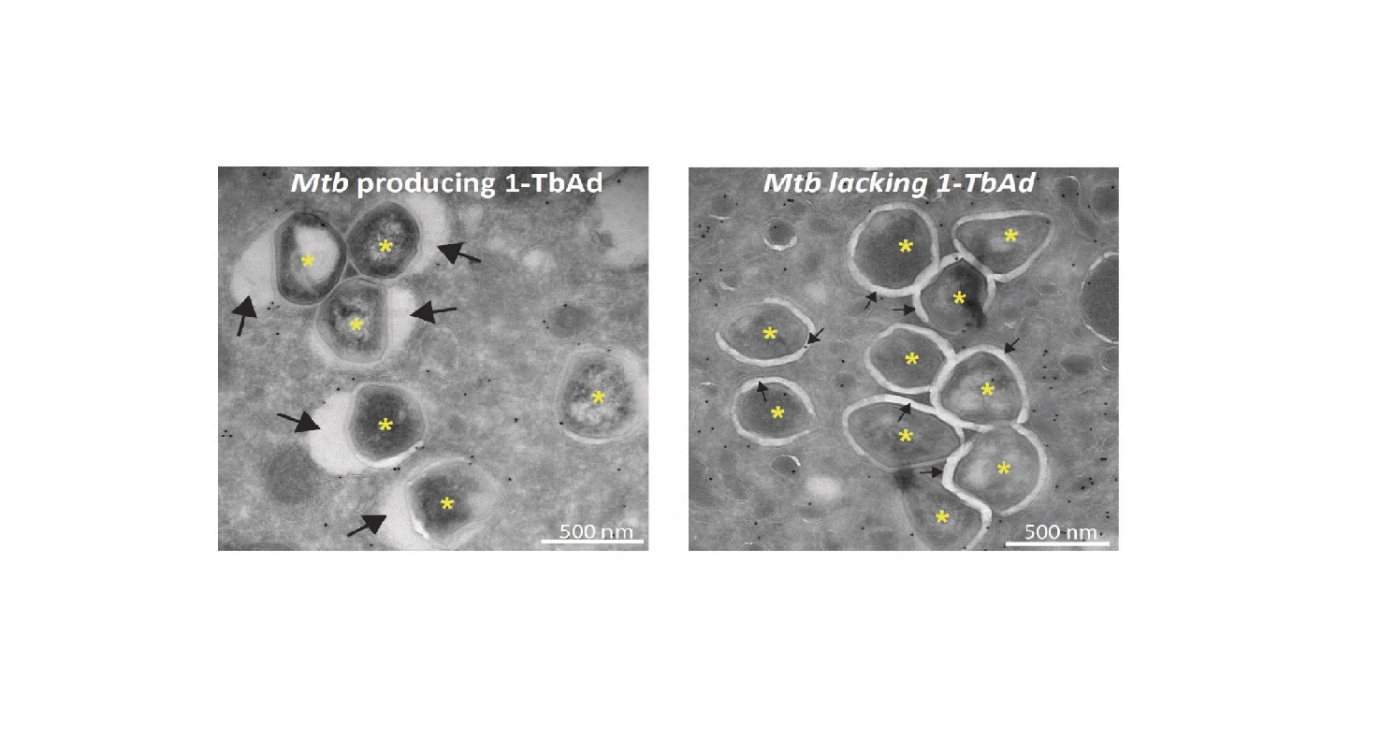
Microscopy studies performed in the lab of Nicole van der Wel at the Amsterdam UMC reveal that exposing macrophages to 1-TbAd makes their lysosomes swell up to five times their normal size. Tests with macrophages infected with M. tuberculosis show that the phagosomes only swell significantly in the presence of the enzyme Rv3378c needed to produce 1-TbAd. ‘There are several mechanisms preventing the macrophages from killing M. tuberculosis, but we discovered what appears to be a key mechanism for bacterial survival’.
Interestingly, the mechanism by which 1-TbAd works is the same as how chloroquine kills the malaria parasite. ‘This drug blocks the functioning of parasite’s lysosomes’. It suggests that 1-TbAd could be used as an anti-malarial drug. ‘But also, targeting the production of 1-TbAd could kill M. tuberculosis inside the macrophages. The enzyme Rv3378c would be an interesting target for drug discovery, as the enzyme is unique to the tuberculosis bacterium’.
Reference: Jeffrey Buter, Tan-Yun Cheng, Marwan Ghanem, Anita E. Grootemaat, Sahadevan Raman, Xinxin Feng, Ashmir R. Plantijn, Thomas Ennis, Joyce Wang, Rachel N. Cotton, Emilie Layre, Alexandrea K. Ramnarine, Jacob A. Mayfield, David C. Young, Amanda Jezek Martinot, Noman Siddiqi, Shoko Wakabayashi, Helene Botella, Roger Calderon, Megan Murray, Sabine Ehrt, Barry B. Snider, Michael B. Reed, Eric Oldfield, Shumin Tan, Eric J. Rubin, Marcel A. Behr, Nicole N. van der Wel, Adriaan J. Minnaard, D. Branch Moody: Mycobacterium tuberculosis releases an antacid that remodels phagosomes. Nature Chemical Biology 19 August 2019 DOI: 10.1038/s41589-019-0336-0
Source: Press Release University of Groningen