Improved treatment options
Significant improvements have been made in treatment options for people with multidrug-resistant or rifampicin-resistant tuberculosis (MDR-/RR-TB). The most recent WHO Guidelines on the treatment of MDR-/RR-TB, released in December 2022, recommended the use of a shorter (6-months), all-oral, effective, treatment regimen containing bedaquiline (Bdq), pretomanid (Pa), linezolid (Lzd) with/or without a fluoroquinolone (moxifloxacin; Mfx) – the so-called “BPaLM/BPaL regimens” under the programmatic conditions as the regimen of choice for eligible patients over the previous 9 – 18 months treatment regimens. These novel regimens have a positive impact on patient adherence, improve treatment completion and treatment success rates as they are shorter, effective with less pill burden, and more tolerable to patients. They have also been shown to be cost-effective. Widespread introduction of these regimens, with patient-centered care, is urgently needed, both by National TB Programmes (NTPs) and by patients.
After successful implementation of the BPaL Operational Research (OR) activities under the LIFT-TB project (Indonesia, Kyrgyzstan, Myanmar, Philippines, Ukraine, Uzbekistan, and Ukraine), TB REACH Wave 7 projects (in Tajikistan and Ukraine), and a direct KNCV-funded project in Nigeria, currently KNCV is technically supporting countries to implement the recently recommended BPaL-based treatment regimens for programmatic use. This includes assistance to develop programmatic implementation plans, updated national guidelines, job aids, training materials, and assisting in ensuring an active drug safety and monitoring system, recording and reporting system, and monitoring and evaluation, are in place.
Easily incorporated
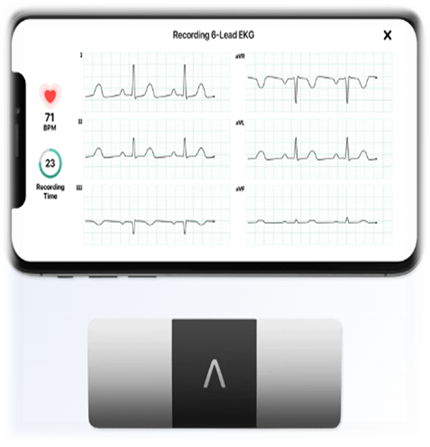 Available generic tools such as the BPaL OR protocol, clinical guide, and related materials, have been updated to be easily incorporated into the national TB guidelines. This can guide countries improve their policies for programmatic implementation of the BPaL(M). Capacity building will provide NTPs with the required skills to implement the regimens. Understanding the importance of patient-centered care, the KNCV programmatic implementation package includes innovations that simplify treatment adherence and safety monitoring, such as digital adherence tools and the use of a portable smart device for ECG monitoring.
Available generic tools such as the BPaL OR protocol, clinical guide, and related materials, have been updated to be easily incorporated into the national TB guidelines. This can guide countries improve their policies for programmatic implementation of the BPaL(M). Capacity building will provide NTPs with the required skills to implement the regimens. Understanding the importance of patient-centered care, the KNCV programmatic implementation package includes innovations that simplify treatment adherence and safety monitoring, such as digital adherence tools and the use of a portable smart device for ECG monitoring.


