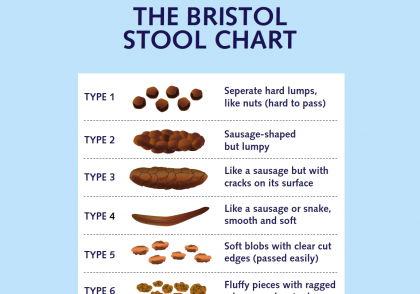
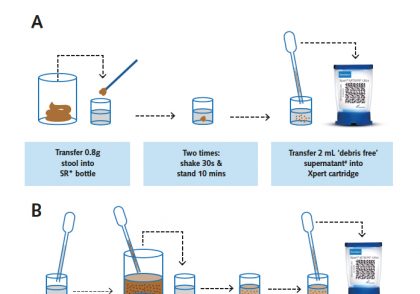
The SOS Stoolbox explained
The SOS Stoolbox is KNCV’s online package to support countries with the implementation of the Simple One-Step (SOS) stool processing method for Xpert MTB/RIF or Xpert MTB/RIF Ultra assay testing for the detection of M. tuberculosis complex (MTBC) in stool. This simple stool processing method is limited to only one release-and-sedimentation step. It makes use of the standard Xpert MTB/RIF or Xpert MTB/RIF Ultra assay test kit for sputum Xpert testing and does not require additional supplies or equipment. The method was validated, and the proof of principle with a demonstration study in routine settings in Ethiopia was published by de Haas & Yenew et al.
The SOS Stoolbox consist of the detailed standard operating procedures (SOP) and list of suggested variables for data collection in English and is now expanded with the following downloadable documents.
DOWNLOAD THE SOS STOOLBOX
Research for optimization using stool as a diagnostic sample and the SOS stool method for stool processing is still ongoing. Therefore, keep an eye on our website as documents are updated whenever new evidence and published data become available. The translations of the Stoolbox SOP in French and Portuguese were kindly funded by USAID/IDDS.
Guidance from WHO on using stool as a primary sample to diagnose TB in people with signs and symptoms of TB
 Since 2020, with an update in 2021, the WHO recommends stool for the diagnosis of TB in children with signs and symptoms of tuberculosis (TB).
Since 2020, with an update in 2021, the WHO recommends stool for the diagnosis of TB in children with signs and symptoms of tuberculosis (TB).
Recently, WHO published the consolidated and operational handbook on tuberculosis Module 5: Management of tuberculosis in children and adolescents. In this guideline, stool is presented as a non-invasive sample for detection TB and is recommended to be used with both Xpert MTB/RIF and the more sensitive Xpert MTB/RIF ultra for children with signs and symptoms of TB. This guideline is accompanied by the GLI practical manual on stool testing in which the SOS stool processing method is recommended as one of the methods to be used to process stool.
Currently, stool testing is not recommended to be used for adults. More evidence is required on the usefulness and acceptability of using stool on adults. However, hopefully more data from a pilot implementation project on the use of stool in routine settings in Vietnam in which the SOS stool test is used for people living with HIV, will be published in the near future.
Training, ongoing studies and webinars
If you want to receive information of additional training materials or training options provided by KNCV on the SOS stool method, please send an email to diagnostics.team@kncvtbc.org.
Stool process videos
Below you will be able to access videos made by FIND on the SOS Stool Method in English, Spanish and Portuguese. The translations of the videos in Ukrainian and French were kindly funded by USAID.